What is a CRO and how can it help you in your clinical trial
What is a CRO
In life sciences, a CRO is an outsourced company that acts on behalf of the sponsor and is in charge of the managing, monitoring and correct development of the clinical study.
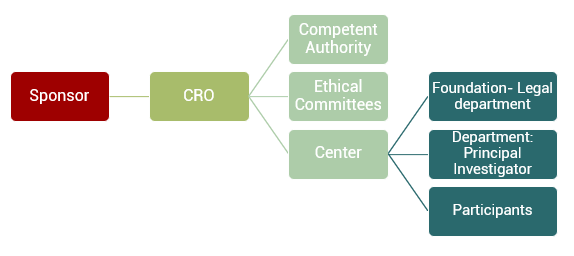
A Contract Research Organization (CRO) acts as a bridge between the sponsor, the one who contracts the services, and the rest of the actors involved in the clinical trial.
It is a type of company that offers its clinical trial management services mainly to the pharmaceutical, biotechnology and medical device manufacturers.
The management of a clinical study is more complicated than it would seem, since many actors intervene (manufacturers, sponsors, ethical committees, Competent Authorities, Centers, Foundations, Researchers, legal departments, participants …). In addition, it is necessary to work under the rules of Good Clinical Practice and the Harmonization Guides (GCP-ICH Guidelines) that ensure the quality of the study. Being able to count on a CRO as a partner in which to trust the management of the study is essential.
Services offered by a CRO
Traditionally, CROs have been in charge of initiating and monitoring clinical trials, but more and more we see companies offering all the services associated with conducting a clinical Study, such are considered “full service CROs”.
The services that a CRO can offer can be divided according to the phase in which we are in the study:
- The start-up includes the development and revision of protocols for trials, the adaptation of the necessary documentation to the applicable legislation, obtaining the necessary approvals from the clinical research ethics committees and regulatory authorities, the design and preparation of the case report forms, the determination of the sample, the selection of the best researchers and research centers and the final negotiation of the contracts.
- Once approval is obtained and the trial begins, the CRO offers its services for monitoring, which consists of controlling compliance with the protocol and with the procedures established for the development of the study. Likewise, pharmacovigilance services include detection and action in the event of the appearance of any Adverse Event.
- The last steps to ensure the success of any clinical study are the management of the data, the generation of reports and the control and storage of the documentation.
- Throughout the study, work is carried out in accordance with Good Clinical Practices (GCPs) that ensure the quality of the study.
Types of CROS
There are different criteria to classify CROs:
- If we take into account the level of specialization of the company, we can find companies specialized in a type of study (clinical trials, research with medical devices or observational studies) and also CROs specialized in a therapeutic area, such as oncology or ophthalmology.
- Taking into account the geographical area in which they move, we can classify CROs as local or global. Global companies tend to be large companies with headquarters all over the world, their coverage is greater, but they are usually less flexible than local companies, whose knowledge of the country’s peculiarities is greater, but their coverage is usually not as extensive.
How to choose a good CRO
Choosing a good CRO is very important, as much of the success of the study will depend on its management and will help the trial run as smoothly as possible.
Some of the questions to ask when choosing it are:
- If you are looking for a CRO that can also advise you on the choice of centers, the number of monitoring and even the necessary sample size. In this second case, it is essential to find an experienced CRO.
- If the way of working adapts to that of the sponsor
- If the rates you propose are adequate and provide a clear and disaggregated budget
- If it is a company committed to the study
Conclusion
CROs are a fundamental part of clinical trials that offer a wide variety of services associated with conducting the study that facilitate the work of the Sponsor.
There are many types of CROs, so it is essential that we know what to expect from them before starting to work together because they will be a key partner in the success of the study.