Che cos’è una CRO e come può aiutarvi nel vostro studio clinico
CHE COS’È UNA CRO
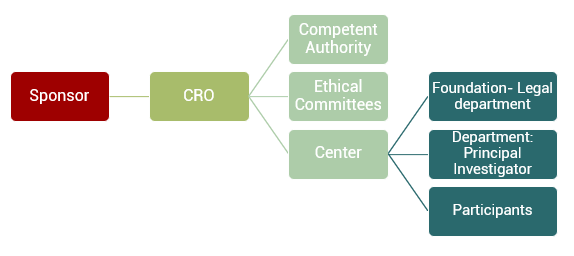
Una CRO è un tipo di società che fa da ponte tra lo sponsor, che contratta i servizi, e il resto degli attori coinvolti nella realizzazione dello studio clinico. La CRO è un’organizzazione di ricerca a contratto (CRO).
Un’organizzazione di ricerca a contratto (CRO) funge da ponte tra lo sponsor, colui che contratta i servizi, e il resto degli attori coinvolti nella sperimentazione clinica.
È un tipo di società che offre i suoi servizi di gestione degli studi clinici principalmente ai produttori di farmaci, biotecnologie e dispositivi medici.
La gestione di uno studio clinico è più complicata di quanto sembri, poiché intervengono molti attori (produttori, sponsor, comitati etici, autorità competenti, centri, fondazioni, ricercatori, uffici legali, partecipanti…). Inoltre, è necessario lavorare secondo le regole della Buona Pratica Clinica e le Guide di Armonizzazione (GCP-ICH Guidelines) che garantiscono la qualità dello studio. Poter contare su una CRO come partner a cui affidare la gestione dello studio è essenziale.
Services offered by a CRO
Traditionally, CROs have been in charge of initiating and monitoring clinical trials, but more and more we see companies offering all the services associated with conducting a clinical Study, such are considered “full service CROs”.
The services that a CRO can offer can be divided according to the phase in which we are in the study:
- The start-up includes the development and revision of protocols for trials, the adaptation of the necessary documentation to the applicable legislation, obtaining the necessary approvals from the clinical research ethics committees and regulatory authorities, the design and preparation of the case report forms, the determination of the sample, the selection of the best researchers and research centers and the final negotiation of the contracts.
- Once approval is obtained and the trial begins, the CRO offers its services for monitoring, which consists of controlling compliance with the protocol and with the procedures established for the development of the study. Likewise, pharmacovigilance services include detection and action in the event of the appearance of any Adverse Event.
- The last steps to ensure the success of any clinical study are the management of the data, the generation of reports and the control and storage of the documentation.
- Throughout the study, work is carried out in accordance with Good Clinical Practices (GCPs) that ensure the quality of the study.
Types of CROS
There are different criteria to classify CROs:
- If we take into account the level of specialization of the company, we can find companies specialized in a type of study (clinical trials, research with medical devices or observational studies) and also CROs specialized in a therapeutic area, such as oncology or ophthalmology.
- Taking into account the geographical area in which they move, we can classify CROs as local or global. Global companies tend to be large companies with headquarters all over the world, their coverage is greater, but they are usually less flexible than local companies, whose knowledge of the country’s peculiarities is greater, but their coverage is usually not as extensive.
How to choose a good CRO
Choosing a good CRO is very important, as much of the success of the study will depend on its management and will help the trial run as smoothly as possible.
Some of the questions to ask when choosing it are:
- If you are looking for a CRO that can also advise you on the choice of centers, the number of monitoring and even the necessary sample size. In this second case, it is essential to find an experienced CRO.
- If the way of working adapts to that of the sponsor
- If the rates you propose are adequate and provide a clear and disaggregated budget
- If it is a company committed to the study
Conclusion
CROs are a fundamental part of clinical trials that offer a wide variety of services associated with conducting the study that facilitate the work of the Sponsor.
There are many types of CROs, so it is essential that we know what to expect from them before starting to work together because they will be a key partner in the success of the study.