Contract Research Organizations – CRO in Portugal
We have prepared a list of the main Contract Research Organizations – CRO in Portugal as well as some useful information regarding those CROs.
We hope it is interesting for you and if any of the below information may have changed or is there any other CRO in Portugal, that is not listed, please don’t hesitate to contact us in order to include the information provided.
List of main CROs in Portugal:
- Leon Research
- Aibili
- Blueclinical
- Cetera
- Labcorp Drug Development (Before Covance)
- CTI Clinical Trial and Consulting Services
- IQVIA
- Keypoint
- Nova-cru
- PhD Trials
- Pivotal
- PPD
- Scietific toolbox consulting
- Syneous Health
- Vectorb2b
- W4Research
Contact information:
Contact page
Phone: +34 987 261 064
Location of the CRO in Portugal:
Rua de Ceuta, N 118-1º 4050-190 Porto (Portugal)
Company size: 11 – 50 employees
Headquarters: León, Spain
Founded: 2007
Specialties: Early Phases Clinical Trials, Observational studies, Ophthalmology Clinical Studies, Phase III Clinical Trials, Food studies, Regulatory and EC approval, Pharma Outsourcing, Clinical studies monitoring, Data Management, Pharmacovigilance
Contact information:
Contact page
Location of the CRO in Portugal:
Edifício Prof. Doutor José Cunha-Vaz
Azinhaga Sta. Comba, Celas
3000-548 Coimbra, Portugal
Company size: 51-200 employees
Headquarters: Coimbra, Portugal
Founded: 1989
Specialties:
Clinical Research, Academic CRO, Clinical Trials, Investigator logistical support, Ophthalmology Reading Centre, Ophthalmology Imaging Technologies, Health Technology Assessment, Market Access, Drug Safety, and Data Centre
Contact information:
Contact page
Location of the CRO in Portugal:
Avenida Villagarcia de Arosa 1919, 1º
4460-439 Senhora da Hora, Matosinhos
Portugal
Company size: 51-200 employees
Headquarters: Matosinhos, Portugal
Founded: 2012
Specialties:
Phase I Studies in Healthy Volunteers, Early Stage Studies in Selected Populations of Patients , Consultancy Services in Drug Clinical Development, and Full-service CRO for Clinical Studies in Patients
Do you want to know what are the main mistakes that we usually make when starting a clinical study?

Fill out this form and we will send you our micro-ebook:
Contact information:
Contact page
Location of the CRO in Portugal:
AIDFM Av. Prof. Egas Moniz, Piso 01
1649-028 Lisbon
Portugal
Company size: 11-50 employees
Headquarters: Lisbon, Portugal
Founded: 2013
Specialties: Scientific Consulting & Advisory, Medical Writing & Translation, Clinical Study Management & Monitoring, and Regulatory Affairs
Contact information:
Contact page
Location of the CRO in Portugal:
Galerias Alto da Barra
Avenida das Descobertas, 59 – Piso 3
2780-053 Oeiras
Portugal
Company size: More than 10,000 employees
Headquarters: Burlington, North Carolina, United States
Founded: 1996
Specialties:
Drug Development Services, Toxicology Services, Central Laboratory Services, Clinical Trial Management Services, Clinical Research, Phase I-IIa, Phase II-III, Preclinical Solutions, Cardiovascular, Oncology, Market Access, Rare Disease, Orphan Drugs, Biotech, Pharmaceuticals, Crop Protection, Clinical Trial Optimization, Market Access, Clinical Trials, Biologics, Cell and Gene Therapy, Pharmacovigilance, NASH, Infectious Diseases, Drug Testing, CRO, Immuno-Oncology, Commercialization, Pediatrics, Kidney Disease, Inflammation, Life Sciences, Bioassays, Bioanalysis, Biologics, Large Molecule, and Small Molecule
Contact information:
Contact page
Location of the CRO in Portugal:
Rua Tierno Galvan, Torre 3, Piso 16, Lisbon, Portugal
Company size: 5001-1,000 employees
Headquarters: Covington, KY, United States
Founded: 1999
Specialties: Feasibility, Regulatory Affairs Study Start-Up, Clinical Project Management, Clinical Monitoring, Medical Monitoring, Safety & Pharmacovigilance, Biometrics, Quality Assurance, Clinical Systems, Training & Development
Contact information:
Location of the CRO in Portugal:
Lagoas Park
Edifício 3 – Piso 3
2740-266 Porto Salvo, Portugal
Company size: More than 10,000 employees
Headquarters: Danbury, CT and Durham, NC, United States
Founded: 2016
Specialties: echnology, Consulting, and Clinical Development
Contact information:
Location of the CRO in Portugal:
Edifício Premium Espaço
Al. Fernão Lopes, nº16A
7º Andar, Escritório 1
1495-190 Miraflores, Portugal
Company size: 11-50 employees
Headquarters: Miraflores, Angés, Portugal
Founded: 1999
Specialties: Health, Epidemiological Studies, Clinical Trials, Observacional Studies, Biostatistics, Medical Writing, Data Management, Registry studies, Advisory Boards, and Health Communication
Contact information:
Contact page
Location of the CRO in Portugal:
CEDOC – Centro de Doenças Crónicas da NOVA Medical School
Rua do Instituto Bacteriológico, nº 5, 5-A e 5B
Edificio Amarelo – 2º piso
1150-190 Lisbon, Portugal
Company size: 11-50 employees
Headquarters: Lisbon, Portugal
Specialties: Protocol Development, Clinical Trial Management, ICH-GCP Monitoring and Medical Monitoring, Medical Writing, Clinical Trial Submission, Pharmacovigilance, Data Management, Biostatistics
Contact information:
Contact page
Location of the CRO in Portugal:
Clinical Center
Avenida Maria Helena Vieira da Silva, nº 24 A – 1750-182 Lisbon, Portugal
Head Office
Rua do Centro,23- 2240-300 Casal de Sta Iria, Chãos, Ferreira do Zêzere, Portugal
Company size: 11-50 employees
Headquarters: Lisbon, Portugal
Founded: 2011
Specialties: Cosmetic trials, Efficacy studies, Safety studies, In vivo, and Raw materials
Contact information:
Location of the CRO in Portugal:
Rua Prof. J. Mineiro 16-1A
2730-146 Lisbon,
Portugal
Company size: 51-200 employees
Headquarters: Madrid, Spain
Founded: 2001
Specialties: oncology, Regulatory Affairs, Clinical Operations, Patient Journey, Data Management,
Biostatistics, Medical Services,
Pharmacovigilance,
Quality Assurance
Contact information:
Contact page
Location of the CRO in Portugal:
Rua Prof. J. Mineiro 16-1A
2730-146 Lisbon,
Portugal
Company size: More than 10,000 enmployees
Headquarters: Wilmington, NC, United States
Founded: 1985
Specialties: clinical research, drug development, CRO, patient recruitment, laboratory, pharmaceutical, biotechnology, biopharma, biopharmaceutical, consulting, therapeutics, Functional Service Partnerships, consulting, Early Development, Post-Approval, medical writing, drug information, pharmacovigilance, biostatistics, and bioanalytical
Contact information:
Contact page
Location of the CRO in Portugal:
Head Office: Rua Dr. Afonso Cordeiro, nº 877 – sala 201 4450-007 Matosinhos, Portugal
Office (Lisbon): LISPOLIS – Centro de Investigação e Desenvolvimento (CID) Estrada do Paço do Lumiar 44, Lote 01 1600-546 Lisbon, Portugal
Company size: 2 -10 employees
Headquarters: Matosinhos, Portugal
Founded: 2012
Specialties: Medical Writing, Clinical Data Management, Biostatistics, Contract Research Organization, Investigator Initiated Research, Study Start-Up, Clinical Operations, Clinical Research, Clinical Trials, Epidemiology, CDISC, SAS Programming, Scientific Consultancy, Medical Translations, Medical Communication, Training in Clinical Research, Statistical Programming, SDTM, Clinical Monitoring, and Regulatory Start-Up (in Portugal)
Contact information:
Contact page
Location of the CRO in Portugal:
Av. da Liberdade, 258, 6º Andar | 1254-149
Lisbon (Portugal)
Company size: More than 10,000 employees
Headquarters: Morrisville, NC, United States
Founded: 2017
Specialties: CRO, Clinical Trials, and Commercialization
Contact information:
Contact Page
Location of the CRO in Portugal:
Ave. D. João II, 42, Building Mythos, Office 204 Parque das Nações 1990-095 Lisbon, Portugal
Company size: 11- 50 employees
Headquarters: Lisbon, Portugal
Founded: 2019
Specialties: GMP production, Biotech, Pharmaceutical, Toxicity, Drug Discovery, Drug Development, Efficacy Assays, Process Development, GMP Production, Biologics, Clinical Trials, Project Management, Antibodies, Scale Up, and Health
Contact information:
Contact page
Location of the CRO in Portugal:
Av. Fontes Pereira de Melo, 31, 7ºB, Lisbon, 1050-117, Portugal
Company size: 11- 50 employees
Headquarters: Lisbon, Portugal
Founded: 2014
Specialties: Clinical Studies Development & Monitoring, Clinical Trials & Non-interventional Studies, Statistics, Medical Writing, Data Management & Programming, Clinical Research & Behavioural Training, Resourcing, Pharmacovigilance, Advisory Boards, and Marketing Consulting
Main reasons to chose Portugal to conduct your clinical studies
We know what is important for you when choosing the countries for the development of your clinical trial. So, we would like to share with you some important data about Portugal so you can decide if it fits your needs.
1. High quality teams
The first thing you should know about Portugal is that it is a small country full of talents. There you can find very competent investigators supported by highly qualified teams able to provide quality data.
2. Target population
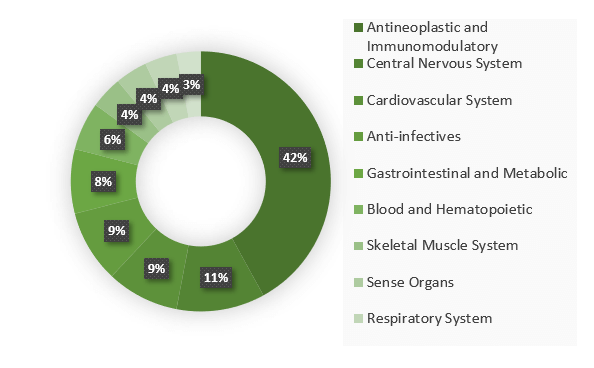
If you are wondering if Portugal has the target population you are looking for, we can tell you the experts believe that oncology, neurology, rare diseases and advanced therapy are the therapeutical areas with more potential to grow in Portugal in the next few years, either because of the high competence of the Portuguese researchers or due to the low number of patients needed.
In the graph you can check the percentage of clinical trials submitted in Portugal by therapeutic area between 2014 and 2017 according to the report of PWC “Ensaios Clínicos em Portugal”.
3. Enrolment
In Portugal, patients are very receptive to be enrolled in clinical trials due to the good relationship they have with clinical investigator. This relationship increases patient’s confidence and therefore retention of recruited patients. However, due to the inexistence of an integrated system to disclose clinical trials taking place inside and outside of the institutions, results in slow and reduced recruitment rates.
4. Approvals
Typically, the speed of approval is one of the main criteria taken into account by the pharmaceutical industry to select a country to carry out their clinical trial. The perception of the industry is that Portugal does not have an approval time as competitive as other European countries.
In 2019 the CEIC (EC) needed on average 71.1 working days to issue the final opinion while INFARMED (CA) needed 23 working days to issue the final decision.
If you think that Portugal fulfils the requirements to receive your clinical trial, you can of course contact us to help you in all the steps to implement your clinical trial in Portugal or you can also check the complete list of CROs with offices in Portugal that we prepared for you.